O-linked glycosylation
O-glycosylation is so diverse that it would be more exact to refer to it at the plural form, so speaking of O-glycosylations. Common to all types of O-glycosylation is the linkage of carbohydrates to the hydroxyl group of acceptor amino acids like serine, threonine and hydroxylysine. The subclasses of O-glycosylation are defined by the first monosaccharide transferred, thereby introducing O-mannosylation, O-fucosylation and so on. These monosaccharides occur in both a and b anomerism, so that the dedicated core glycosyltransferases have different topologies and structural features. As discussed in more details later, the initiation of O-glycosylation is heterogeneous. Some core glycosyltransferases are localized in the ER, whereas others are in the Golgi apparatus.
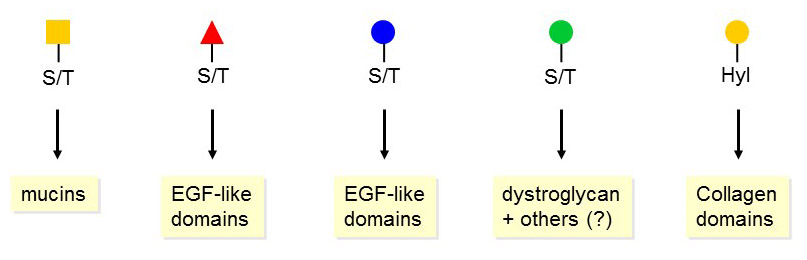
Figure 45. Core structures of O-glycoslyation in animals.
Another major difference between N- and O-glycosylations relates to the types of glycoproteins modified. N-glycosylation is pleiotropic, but most subclasses of O-glycosylation are specific for some kind of peptidic domains or types of proteins. For example, O-GalNAc glycans are often found clustered in mucin domains, while O-fucosylation specifically takes place on EGF-like and thrombospondin domains. All core monosaccharides, with the exception of O-Gal, are transferred to the accepting amino acids serine and threonine. O-Gal is attached to hydroxylysine, which is found in collagen domains.