O-Fucosylation and O-glucosylation
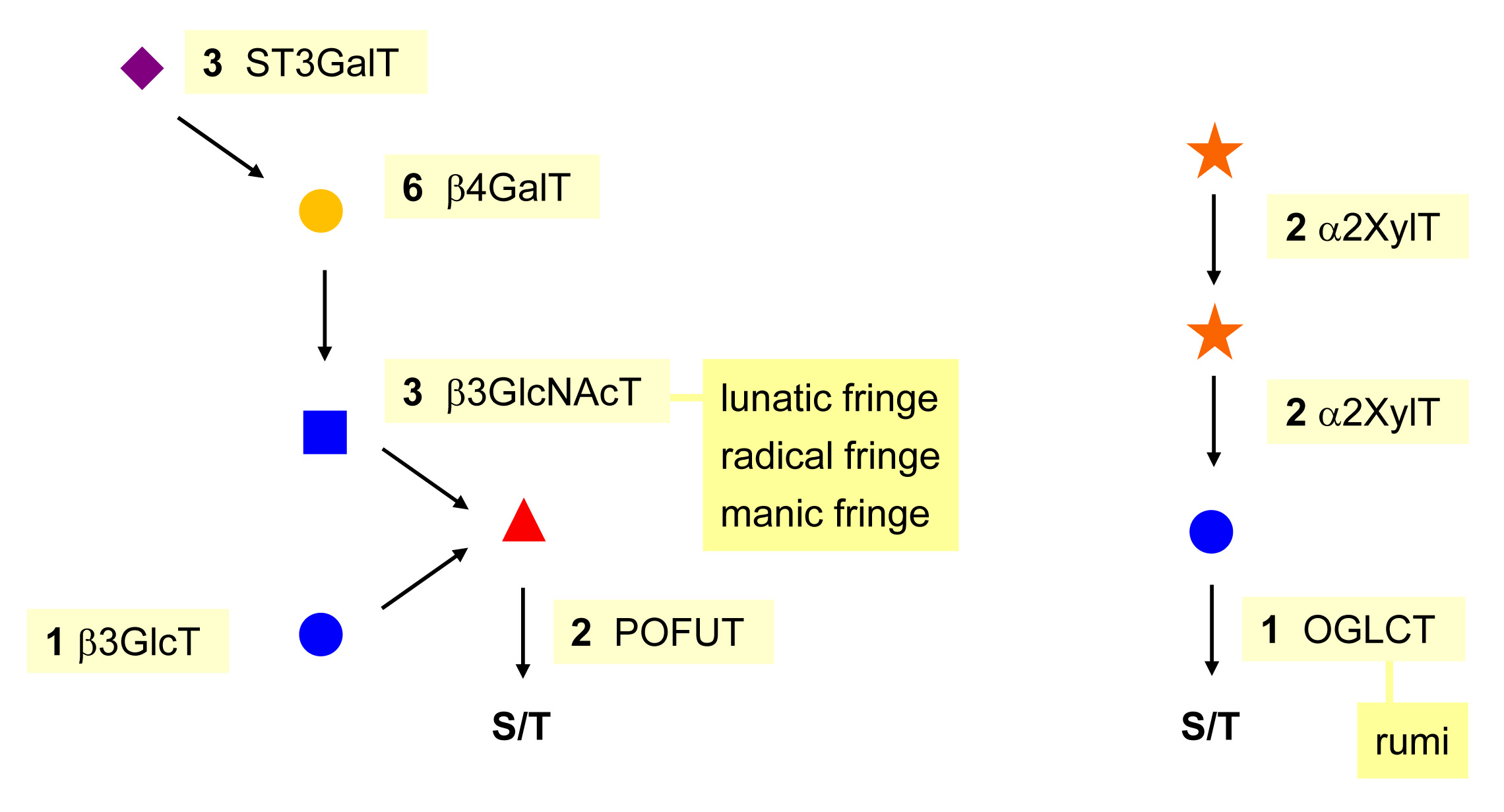
O-Fucosylation and O-glucosyaltion are specialized types of O-glycosylation targeting epidermal growth factor (EGF)-like domains and thrombospondin type 1 (TSP1) domains. The transfer of Fuc and Glc to selected serine and threonine residues on EGF-like and TSP1 domains takes place in the ER and uses GDP-Fuc and UDP-Glc as donor substrates. These forms of O-glycosylation occur in animals and they have been first characterized in Drosophila. This work in Drosophila explains the exotic names given to some of the glycosyltransferases involved in O-fucosylation and O-glucosylation, such as Fringe (β1-3 GlcNAc-transferase) and Rumi (protein O-Glc-transferase).
The EGF-like domain consists of a cysteine-rich stretch of about 40 amino acids, which usually form three disulfide bridges. EGF-like domains are often found as multiple repeats in proteins. For example, the Notch receptor has 36 EGF-like domains and its ligands Delta1 and Jagged1 have respectively 8 and 16 EGF-like domains. Other proteins like coagulation factors and signaling proteins also contain EGF-like domains that are likely to be O-glycosylated.
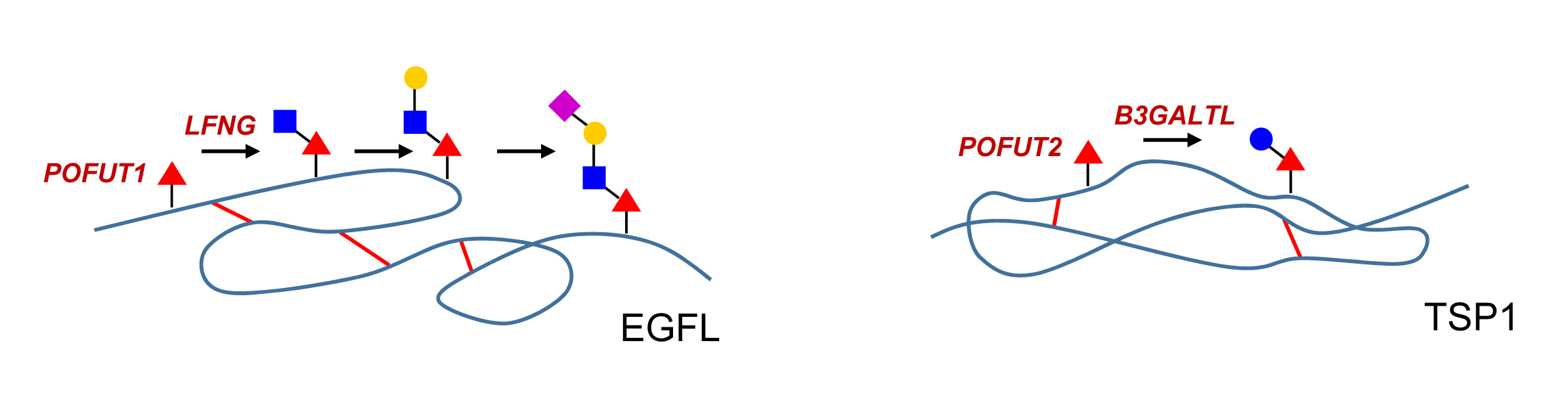
The consensus site for O-fucosylation is loosely defined by an acceptor serine/threonine site flanked by cysteine residues. These cysteines need to be in the context of disulfide bridges to allow the recognition of the EGF-like domain by the protein O-Fuc-transferase enzyme OFUT1. A second protein O-Fuc-transferase (OFUT2) enzyme exists in animals and directs the transfer of Fuc to TSP1 repeats. The TSP1 domain encompasses about 50 amino acids and contains, like the EGF-like domain, six cysteine residues building three disulfide bridges. TSP1 repeats have been first described in thrombospondin but they also occur in serum proteins of the complement pathway, e.g. properdin, C6, and C7. The inactivation of the Ofut1 and Ofut2 genes in mice leads in both cases to embryonic lethality.
The addition of β1-3 GlcNAc on O-Fuc is mediated by Fringe, a glycosyltransferase first described in Drosophila as a modifier of Notch. Vertebrates have three isoforms, which are called lunatic, manic and radical Fringe. The β1-4 Gal-transferases and α2-3 Sia-transferases elongating O-Fuc glycans on EGF-like repeats are the same as the enzymes involved in the elongation of N- and O-GalNAc glycans.
Some EGF-like repeats contain O-Fuc and O-Glc glycans simultaneously. The exact roles of these glycans on glycoproteins are unclear. In the context of Notch and its ligands Delta and Jagged (called Serrate in Drosophila), it has been shown that at least the disaccharide GlcNAc(β1-3)Fuc is required for ligand binding. By contrast, O-Glc glycans do not appear to be involved in ligand binding but rather in stabilizing glycoproteins. In fact, mutations in Rumi lead to a temperature-sensitive Notch phenotype in Drosophila.
The functional study of O-fucosylated and O-glucosylated glycans in Notch biology is difficult considering the heterogeneous glycosylation of the 36 EGF-like repeats of Notch. Note worthily, most EGF-like repeats bind Ca2+, thereby increasing the rigidity of Notch. Some repeats, like #11 and #22 do not bind Ca2+and remain unglycosylated. It has been postulated that Notch can bend at these unglycosylated repeats and fold around its ligands for stronger interactions. NMR studies will be necessary to confirm this concept, although this will be quite a challenging task considering the large size and complex glycosylation pattern of Notch.
Mutations of the β1-3 Glc-transferase gene acting on TSP1-linked O-Fuc cause Peters-Plus syndrome. The disease is characterized by psychomotor and growth retardation, where adult patients have short stature and usually do not exceed 1.5 m. A typical feature of Peters-Plus syndrome is keratolenticular adhesion, which is a caused by a developmental defect of the lens and anterior eye chamber. However, the mechanisms and the O-fucosylated glycoprotein involved in this abnormal development are still unknown. The role of O-fucosylation in mammalian development is also underlined by the skeletal defects resulting from abnormal vertebral segmentations. Accordingly, mutations in the Lunatic fringe gene (LFNG) and in the Delta-3 gene (DLL3) have been described in such cases of spondylocostal dysostosis.