Structural features
As defined by the International Union of Pure and Applied Chemistry, glycans are structures of multiple monosaccharides linked through glycosidic bonds. The terms sugar and saccharide are synonyms, depending on your preference for Arabic (“sukkar”) or Greek (“sakkēaron”). Saccharide is the root for monosaccharides (a single carbohydrate unit), oligosaccharides (3 to 20 units) and polysaccharides (large polymers of more than 20 units).
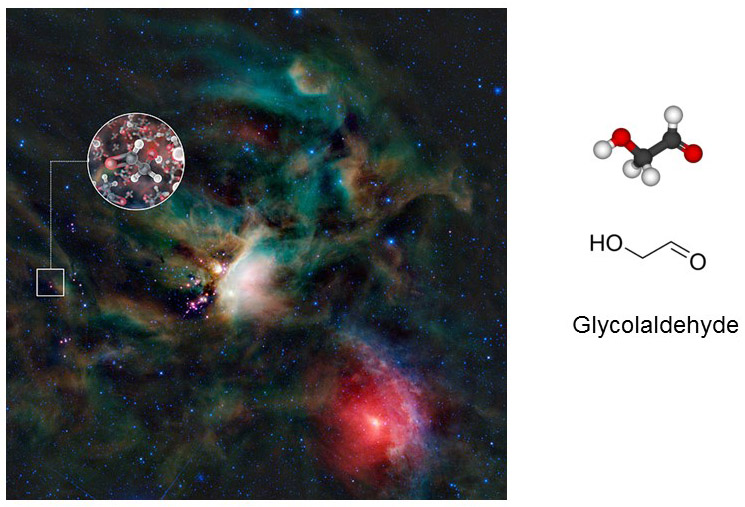
Carbohydrates follow the basic formula (CH2O)N>2. Glycolaldehyde (CH2O)2 would be the simplest member of the family if molecules of two C-atoms were not excluded from the biochemical repertoire. Glycolaldehyde has been found in space in cosmic dust surrounding star-forming regions of the Milky Way galaxy. Glycolaldehyde is a precursor of several organic molecules. For example, reaction of glycolaldehyde with propenal, another interstellar molecule, yields ribose, a carbohydrate that is also the backbone of nucleic acids.
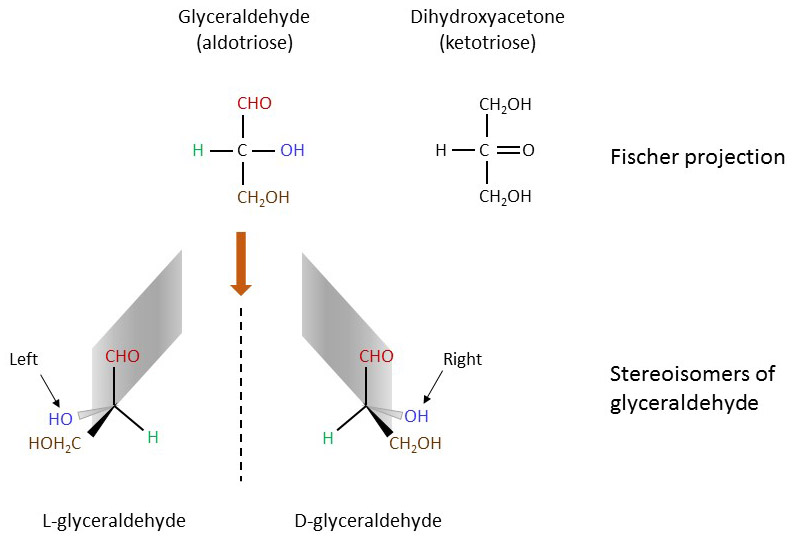
Beginning the count at three carbon atoms, glyceraldehyde and dihydoxyacetone share the common chemical formula (CH2O)3 and represent the smallest carbohydrates. As their names imply, glyceraldehyde has an aldehyde group (at C1) and dihydoxyacetone a carbonyl group (at C2). Carbohydrates with an aldehyde group at C1 like glucose are called aldoses and those with a carbonyl group at C2 like fructose are called ketoses. Glyceraldehyde exists as two mirror image isomers (D- and L-stereoisomers), whereas dihydroxyacetone has no chiral carbon. Figure 3. Chirality of glyceraldehyde.
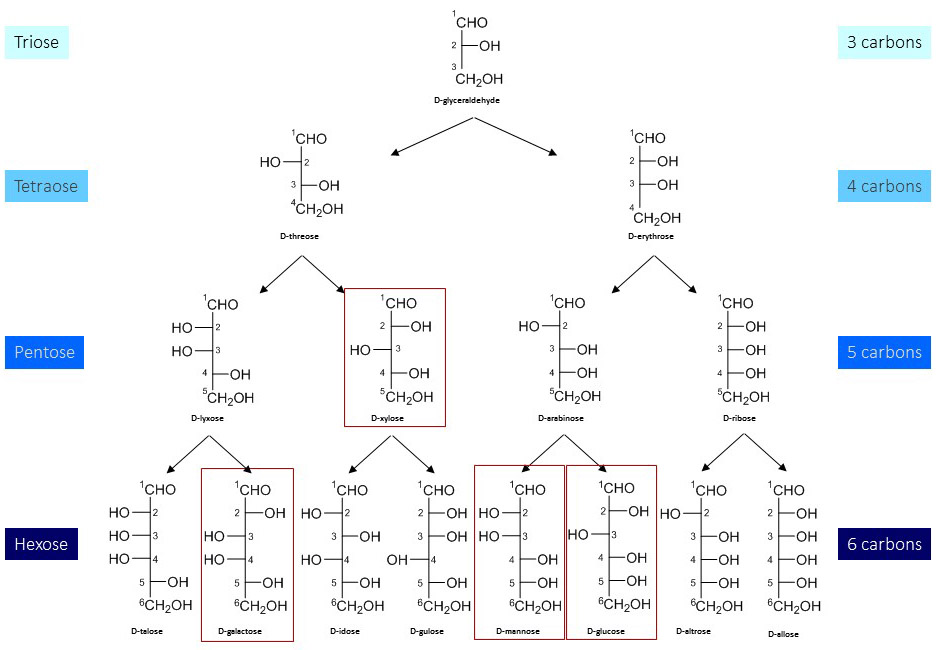
The distinction between D- and L-stereoisomers of carbohydrates is based on the orientation of the asymmetrical C-atom the farthest away from the carbonyl terminus (i.e. C5 in hexoses, see below). If the hydroxyl group at this asymmetrical center is oriented to the right (like D-glyceraldehyde in the Fischer projection), the carbohydrate becomes a D-stereoisomer. Most monosaccharides have at least one chiral C-atom with the total number (k) being equal to the number of internal CH2O groups, i.e. n-2 for aldoses and n-3 for ketoses with n being the number of C-atoms in the monosaccharide. Thus, the possible number of stereoisomers in a monosaccharide is equal to 2k. Accordingly, there are eight possible stereoisomers for hexoses. Figure 4. Stereoisomers from trioses to hexoses.
Two new stereoisomers are introduced with each new carbon center added. If this new center is virtually introduced between C1 (carbonyl group) and the previous C2 center, the stereoisomerism (D or L) of the new monosaccharide is maintained. Monosaccharides are grouped based on their number of C-atoms, thus yielding trioses, tetraoses, pentoses, hexoses, etc. Hexoses are the most abundant monosaccharides in biology, with glucose being at the top of the list. Two monosaccharides that differ only in the configuration of a single chiral C-atom are called epimers. Accordingly, galactose is an epimer of glucose.