Glycosyltransferases
Glycosyltransferases are a group of enzymes that catalyze the transfer of a sugar moiety from an activated sugar onto carbohydrate or non-carbohydrate acceptors. With the exception of hyaluronan synthase, glycosyltransferases elongate glycans by adding monosaccharides to the non-reducing ends of acceptor substrates. The transfer reaction is linkage-specific and the resulting product is fixed in a given anomeric configuration. Glycosyltransferase genes make up a significant portion of prokaryote and eukaryote genomes. Adding all transporters and enzymes required for substrate biosynthesis and for glycan degradation, up to 5% of genomes encode genes involved in glycosylation. Globally, more than 30’000 glycosyltransferases are known to date.
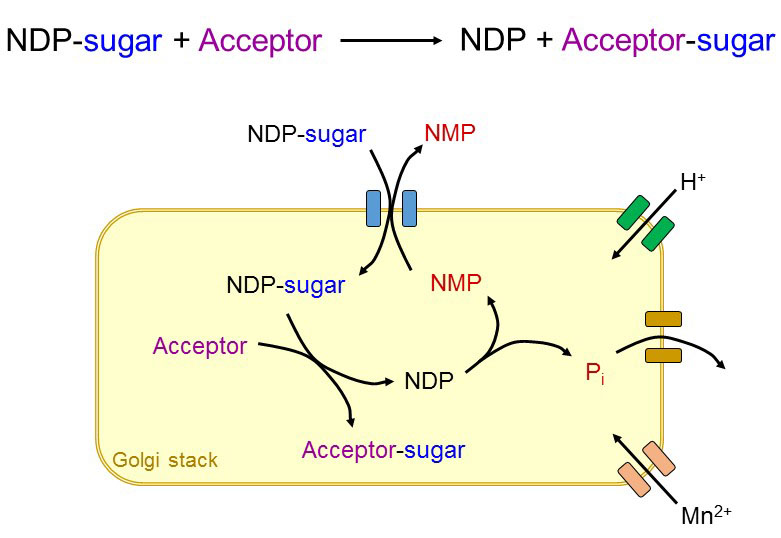
The standard glycosyltransferase reaction requires specific donor and acceptor substrates and yields a glycosylated product and an aglycone, which is typically NDP or a polyprenol-P. Additional parameters like pH, Mn2+ availability and NDP levels affect many glycosyltransferase activities. Therefore, local concentrations of these parameters are strictly controlled in order to maintain proper glycosylation.
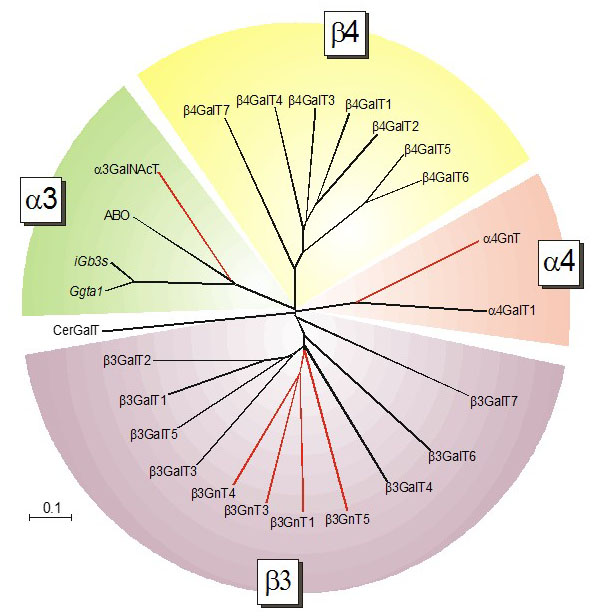
Glycosyltransferases have been first grouped by their substrate specificities. This classification system gave raise to families of galactosyltransferases, fucosyltransferases and sialyltransferases for example, which were further specified according to the type of linkage catalyzed. The broad availability of genomic data in the last decades led to the identification of multiple structurally related proteins for most glycosyltransferase families. As the enzymatic activity of new glycosyltransferases was characterized, it became soon evident that structurally related proteins may indeed catalyze the transfer of distinct donor substrates. Along this line, β1-3 galacatosyltransferases were found to be more similar to β1-3 N-acetylglucosaminyltransferases than to β1-4 galactosyltransferases.
Figure 23. Cladogram of Gal-transferases and structurally related GalNAc- and GlcNAc-transferases (marked with red lines) demonstrating the sequence conservation based on the type of linkage catalyzed.
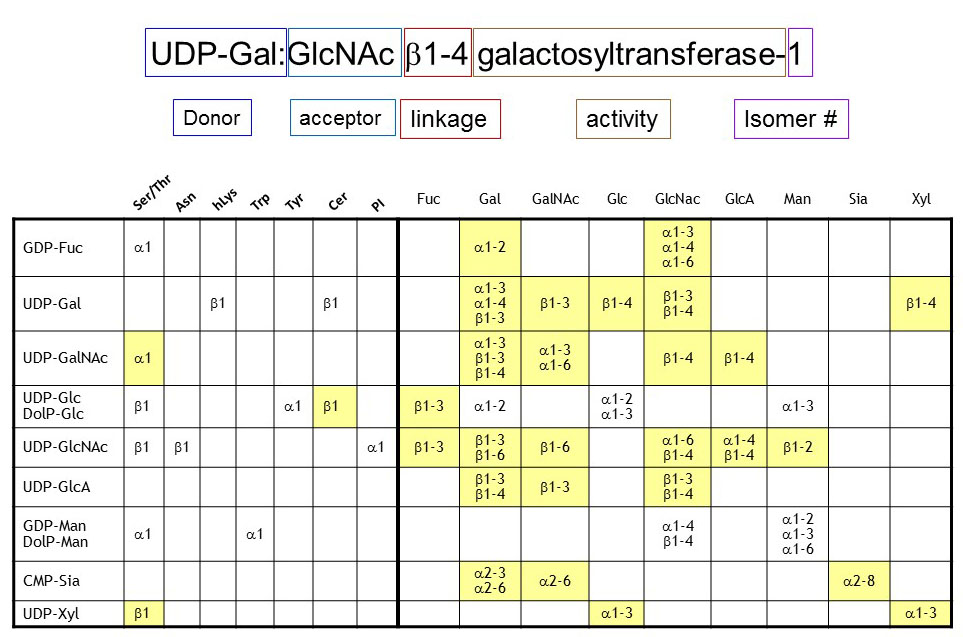
The nomenclature of glycosyltransferases does not only consider the linkage and donor substrate, but also the acceptor substrate and the isomer number are included in the formal name. Nomenclature remains a difficult issue since many glycosyltransferases are usually called by trivial names. For example, a given sialyltransferase is described as SiaT9, GM3 synthase or ST3Gal5 depending on the publications considered. This lack of systematic nomenclature certainly does not simplify the access to glycobiology, which is already complex enough at the biological level.
As mentioned here above, the type of linkage dictates the structural conservation between glycosyl¬transferases. Despite the tremendous amount of combinations between donor, acceptor and linkage exists in theory, only few are really encountered in organisms. For example in animals, the donor GDP-Fuc is only transferred via α1-O linkage to Ser/Thr in the ER and to Gal via α1-2 linkage and to GlcNAc via α1-3, α1-4 and α1-6 linkages in the Golgi apparatus.
Although most glycosyltransferases transfer monosaccharides to a multitude of glycoproteins and glycolipids, some glycosyltransferases recognize large protein domains and thereby are specific to selected types of proteins or even specific to a single protein. This is for example the case for the enzyme GlcNAc-1-phosphotransferase, which initiates the formation of Man-6-P on lysosomal enzymes, or for a GalNAc-transferase acting specifically on gonadotropin hormones.