Glycosyltransferase trafficking
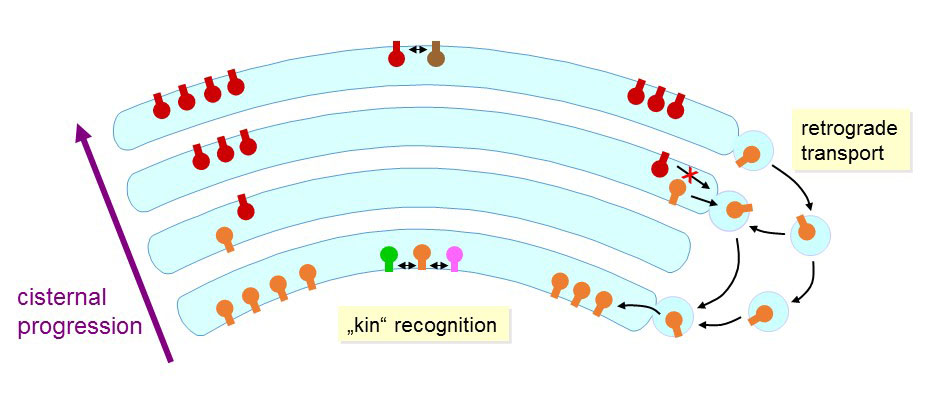
Some Golgi glycosyltransferases are mainly found in cis cisternae whereas others are enriched in the trans cisternae. These gradients are likely the product of selective retrograde transport processes, such as those involving COG tethering factors. The localization of glycosyltransferase may be dictated by signal motifs found in the cytosolic domains, which are exposed to vesicular transport proteins. In fact, localization experiments performed on glycosyltransferases with mutated N-terminal sequences confirmed the presence of such localization signals. In some cases, the simple presence of a di-leucine motif appears to be sufficient for the retention of a glycosyltransferase in the Golgi apparatus.
Another mode of localization has been proposed, which is based on the size of the transmembrane domain of glycosyltransferases and the thickness of Golgi membranes. The cholesterol concentration increases from cis to trans in the Golgi, which increases correspondingly the thickness of the lipid bilayer in which glycosyltransferases are anchored. Some correlations have been documented between the length of the transmembrane domain and the localization of some glycosyltransferases in the Golgi apparatus. However, most glycosyltransferases do not show any correlation of such kind, meaning that the length of the transmembrane domain alone does not account for the localization of glycosyl¬transferase.
Another theory states that the retention of glycosyltransferases is mediated through the formation of heterodimers between enzymes that catalyze successive reactions in a glycosylation pathway. Along the line of this theory of “kin recognition”, it has been shown that the β1-2 GlcNAc-transferase GnT1 interacts with the mannosidase-II enzyme in the medial Golgi. However, experiments with cells from gene knockout mice did not support the kin recognition model, since the absence of a specific glycosyltransferase did not affect the localization of his presumed interacting partners.
Figure 26. Possible control mechanisms of glycosyltransferase localization in the Golgi apparatus.
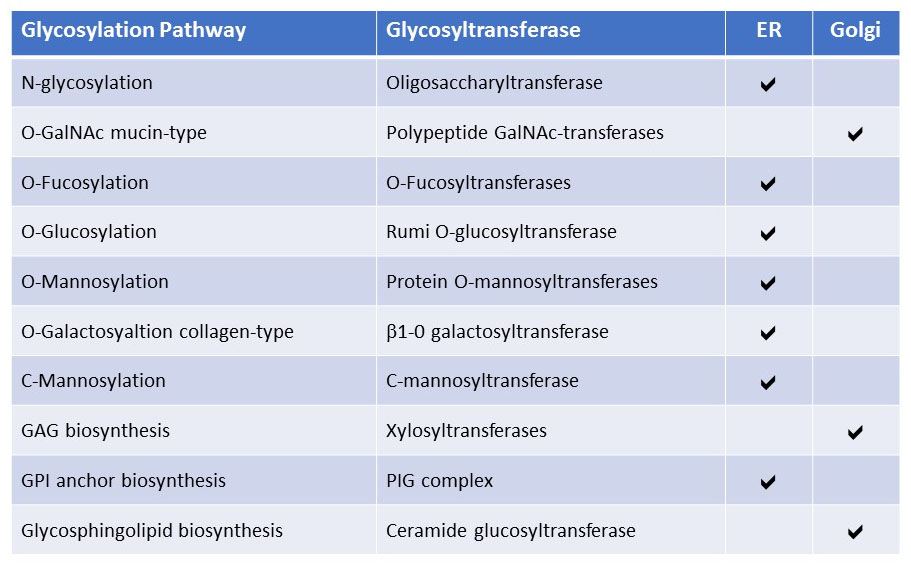
Most core glycosyltransferases that initiate a class of glycosylation are localized in the ER. Only mucin-type glycosylation, GAG chain biosynthesis and glycosphingolipids formation are initiated in the Golgi apparatus based on the localization of their respective core glycosyltransferases. Some of the ER-located core glycosyltransferases transfer glycans to unfolded proteins, like collagen Gal-transferases and the oligosaccharyltransferase complex. Also, the protein O-fucosyltransferase OFUT1 even acts as a chaperone for the folding and trafficking of its substrate glycoprotein Notch.
Figure 27. Initiation of glycosylation pathways in the ER and Golgi apparatus based on the organelle localization of glycosyltransferases.