C-Mannosylation
C-Mannosylation features a unique C-C bond linking C1 of Man to C2 of the indole ring of tryptophan. C-Mannosylation was first described 1994 by Jan Hofsteenge while studying the post-translational modifications of RNase-2. After noticing the presence of an hexose residue covalently linked to tryptophan, Hofsteenge and colleagues identified mannose as the linked carbohydrate and established WxxW as acceptor sequence, in which the first tryptophan is mannosylated. C-Mannosylation takes place in the ER. The donor substrate of C-mannosyltransferase was identified as DolP-Man, which strongly suggested that c-mannosylation takes place in the ER. C-Mannosyltransferase activity is widespread among mammalian cell types. Across phylogeny, the activity is found in most eukaryotes except insects and fungi. Bacteria also appear to lack the activity.
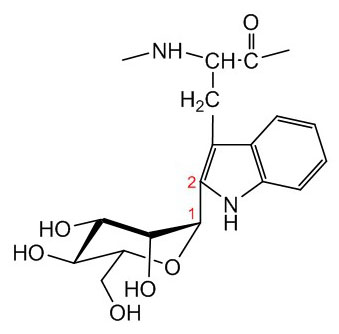
Figure X. C-Mannosylation of tryptophan. Mannose is attached to tryptophan through an a1-2 linkage.
The acceptor sequence WxxW is encountered on multiple proteins and in particular in the context of the thrombospondin type 1 domain. Accordingly, proteins containing this domain, such as complement proteins, semaphorins, ADAMTS, and true thrombospondins are C-mannosylated. Additional proteins carrying C-Man are interleukin 12, the mucin MUC5, and the erythropoietin receptor among others. Some proteins carry multiple C-mannosylated residues. For example, the complement-activating protein properdin has 14 C-mannosylated tryptophan residues. The gene encoding C-mannosyltransferase has only been described in 2013 and shown to be identical to Dpy19, a member of the dumpy mutants in Caenorhabditis elegans. The Dpy19-encoded enzyme was recognized as a candidate glycosyltransferase based on its partial sequence conservation with the DolP-dependent subunit STT3 of the OST complex. Mammalian genomes comprise four Dpy19-related genes designated DPY19L1 to DPY19L4. Mutations in DPY19L2 have been associated with male infertility characterized by globozoospermia. Such spermatozoa have rounded heads, lack acrosomes and thereby cannot fertilize oocytes. The molecular nature of the defect is still unknown as the protein targets of DPY19L2 have not been identified yet. The general function of C-mannosylation is also unclear at this stage, although it has been suggested to affect protein folding and trafficking along the intracellular secretory pathway. In the extracellular space, it is tempting to speculate that C-mannosylation contributes to the mannose-binding lectin (MBL)-mediated activation of complement proteins, which are C-mannosylated.