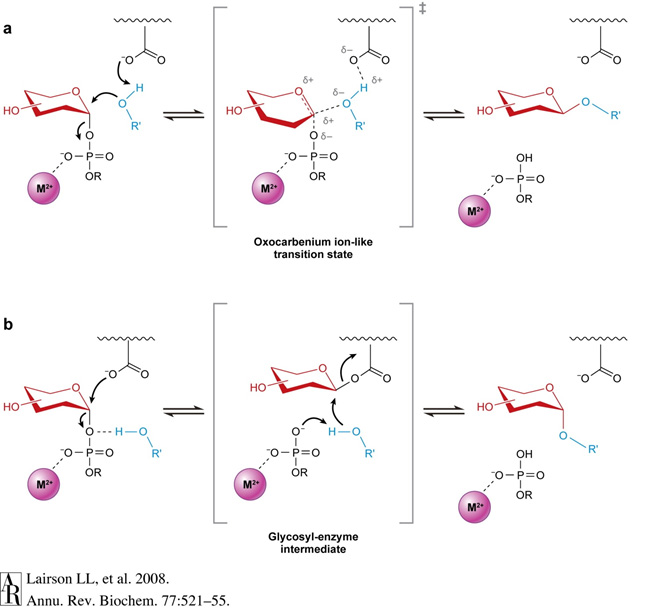
Mechanisms of glycosyltransferase reaction
Most nucleotide-activated sugars are in the α-anomeric form but the products of many glycosyltransferase reactions are in the β-anomeric form. The enzymes catalyzing such reactions are called inverting glycosyltransferases, whereas those maintaining the anomerism in the product are called retaining glycosyltransferases. By the way, sialyltransferases and fucosyltransferases are inverting enzymes, because they catalyze the respective transfer of Sia and Fuc from CMP-β-Sia and GDP-β-Fuc to α-linked products. The catalytic mechanisms of the underlying transfer are totally different between inverting and retaining glycosyltransferases. The mechanism of inverting glycosyltransferases is a direct displacement SN2-like reaction enabled by an enzymatic base catalyst and by Lewis acid activation of the departing phosphate leaving group. The catalytic mechanism of retaining glycosyltransferases is less clear and appears to involve a double-displacement mechanism with formation of a covalently bound glycosyl-enzyme intermediate. The type of glycosyltransferase reaction, i.e. inverting or retaining, does not correlate with the overall structural organization of the enzyme. Both GT-A and GT-B fold topologies have been found in inverting and retaining enzymes.
Figure 28. Mechanisms of inverting (a) and retaining (b) glycosyltransferase reactions. Illustration taken from Lairson LL et al., 2008, Annu Rev Biochem 77:521 ©.
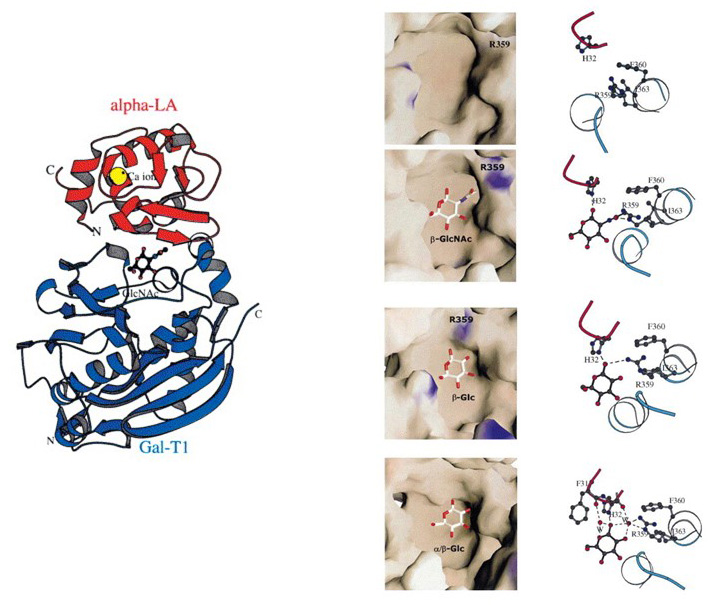
Most glycosyltransferases contain a single catalytic domain but few do comprise two catalytic domains. The latter enzymes are usually involved in the formation of heteropolymers such as glycosaminoglycan chains. At any case, each glycosyltransferase domain catalyzes a single enzymatic reaction. However, as always some exceptions exist to the rule. One of such exceptions deals with the type of linkage, namely the fucosyltransferase enzyme FUT3 catalyzes the transfer of Fuc in both α1-3 and α1-4 linkages. The second exception is the EXTL2 glycosyltransferase that transfers both the sugar donors GalNAc and GlcNAc to GlcA. Finally, the third exception is the β1-4 galactosyltransferase B4GALT1 that transfers Gal to either the acceptor GlcNAc or Glc. Note worthily, the latter reaction requires the association of the B4GALT1 enzyme with the protein α-lactalbumin to encompass the Glc acceptor.
The complex of B4GALT1 with α-lactalbumin is essential for the production of lactose, i.e. Gal(β1-4)Glc, in the lactating mammary gland. The interaction with α-lactalbumin takes place within the catalytic domain B4GALT1, in a region comprising the acceptor-binding site. The proximity of α-lactalbumin changes the orientation of side-chains in a loop of the acceptor-binding pocket of B4GALT1, thereby blocking the binding of GlcNAc and making Glc the preferred acceptor. This conformational change decreases the Km of B4GALT1 for Glc by 1000-fold.
Figure 29. Complex formation between B4GALT1 (blue) and α-lactalbumin (red). Illustration taken from Ramakrishnan and Qasba, 2001, J Mol Biol 310, 205 ©.