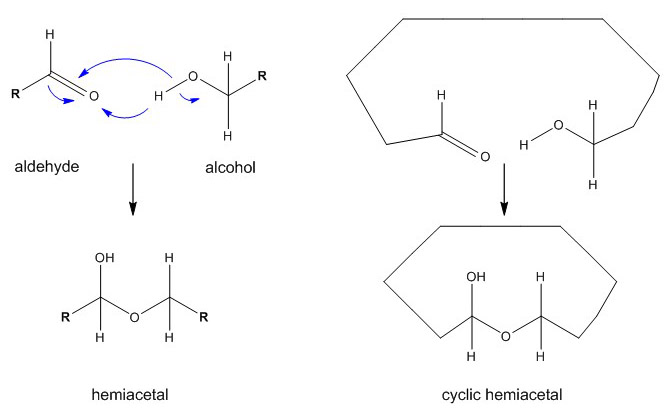
Ring formation
Pentoses and hexoses do not react on tests for free aldehydes because they normally form cyclic structures. The intramolecular condensation between the aldehyde group at C1 and the hydroxyl group of in internal group (mainly C5 or less frequently C4) leads to a ring structure through the formation of a semialdehyde or cyclic hemiacetal. Ketohexoses also build ring structures by forming hemiketals.
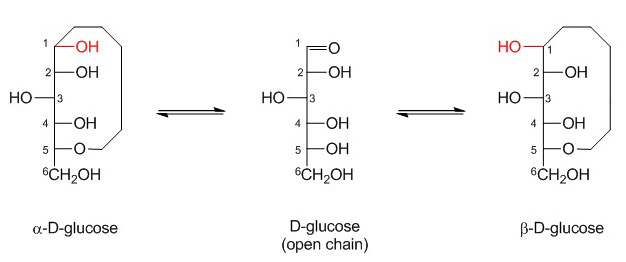
The formation of a ring structure adds a new level of asymmetry, thereby introducing a further isomerization. The new asymmetric carbon is called the anomeric center and a is used to denote the anomer where the absolute stereochemistry of the anomeric position and the most remote stereocenter in the sugar chain are the same. The anomer is called β when the stereochemistry is opposite. For D-monosaccharides, α and β anomers can be identified through the orientation of the hydroxyl group at C1, which is either axial (α) or equatorial (β).
Anomerism has a tremendous impact on the biological properties of carbohydrates. For example, α-D-mannose is perceived as sweet when tasted, whereas β-D-mannose is bitter (Steinhardt et al, 1962, PMID 14037773). Similarly, the α anomers of D-glucose and D-galactose are sweeter than their β anomers, indicating that taste receptors exhibit high stereoisomeric specificity. Carbohydrate anomerism is equally important in the formation of glycosidic linkages as it confers specific physical, chemical, and biological properties to the ensuing products. For example starch and cellulose are both linear polymers of glucose, which only differ by the anomerism of the glycosidic linkage. Cellulose as a β1-4 polymer adopts a stiff linear conformation and forms a near crystalline structure, whereas starch as an α1-4 polymer arranges in a more relaxed structure. Animals can break down starch to Glc since they possess an α1-4 glucosidase activity (amylase) in the saliva and in the intestine. By contrast, animals lack β1-4 glucosidases and thus cannot digest cellulose. If humans could do so, a leaf of salad would provide more calories than a chocolate bar!
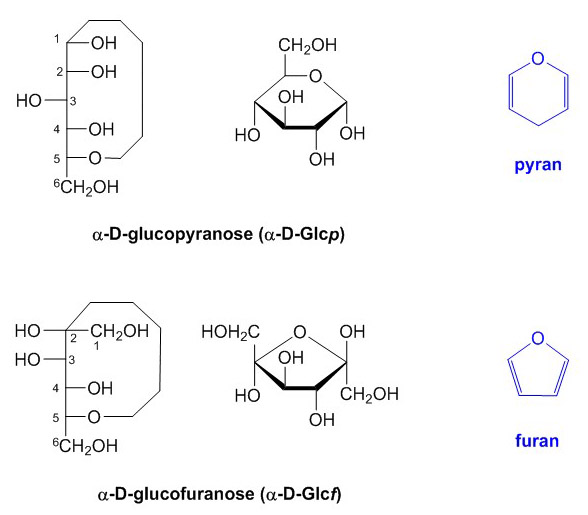
Most hexoses form a 6-atom ring, similar to a pyran ring, hence the term pyranose for such structures. Strictly speaking, the suffix p should be added to the abbreviation of the monosaccharide in question to designate this fact, i.e. Glcp. By analogy, the formation of a 5-atom ring yields furanose based on the 5-atom furan ring.
Figure 7. Pyranose and furanose ring structures of α-D-glucose.
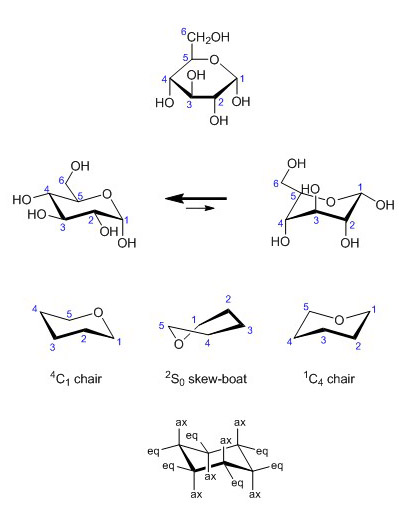
Since pentoses and hexoses form rings, the linear Fischer projection is best replaced by the Haworth representation, which also takes in account the anomerism of carbohydrates. In the conversion from Fischer to Haworth, the groups oriented to the right in Fischer representations are set downwards in Haworth diagrams. The CH2OH group at C6 is shown upwards. The Haworth representation suggests that monosaccharides are flat, but neither furanose nor pyranose rings are actually planar in their lowest energy confirmations. Pyranoses like the cyclohexane ring adopt a low-energy conformation that looks like a chair. The C2-C3-C5-O square forms a plane whereas the C1 and C4 atoms take up a higher or lower position, thus yielding the two possible chairs 1C4 and 4C1.
Figure 8. Chair conformations of α-D-glucose.
Although D-glucose has a strong preference for the 4C1 chair conformation, this is not true for all monosaccharides. For example, L-iduronic acid (an epimer of glucuronic acid) is more stable in the 1C4 configuration. In fact, L-iduronic acid is a special case, because the 1C4 configuration is in equilibrium with the so-called skew-boat shape (2So), which favors the formation of hinges in linear glycan chains.
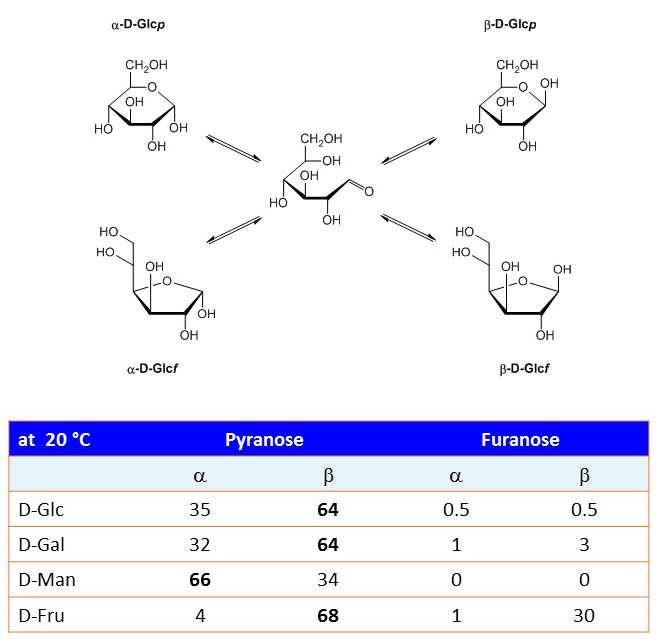
Monosaccharides in solution undergo spontaneous mutarotation that changes the axial (α) and equatorial (β) position of the hydroxyl group at C1. Mutarotation is catalyzed by mild acidic conditions. Although the linear aldehyde form is a negligible fraction of the monosaccharide structure (0.01%), it represents the obligate intermediate in the mutarotation reaction. The aldehyde group of linear glucose can for example react with amino groups of amino acids like lysine and form an N-substituted glycosylamine. This reaction, which is called Maillard reaction, is the first step in the process of non-enzymatic glycation, which results in the formation of advanced glycation endproducts (AGE). Glycation is obviously not to be confused with glycosylation!
Figure 9. Mutarotation of D-glucose and prefered conformations of common monosaccharides in aqueous solution at 20 °C.
Whereas glucose and galactose are predominantly found in the β anomeric form, mannose is most frequently found as α anomer. Why is it so? Look at the orientation of the hydroxyl group at C2.