Mucin-type O-glycosylation
The transfer of O-GalNAc to serine and threonine in mucin domains is the most common type of O-glycosylation encountered in vertebrates. It is important however to mention that this type of O-glycosylation also takes place on proteins that have no mucin domains. The lack of consensus acceptor sequence and the lack of endoglycosidases able to cleave all O-GalNAc glycans (comparable to the PNGaseF enzyme used to cleave N-glycans) make it difficult to predict and verify the occurrence of such O-GalNAc glycans on proteins.
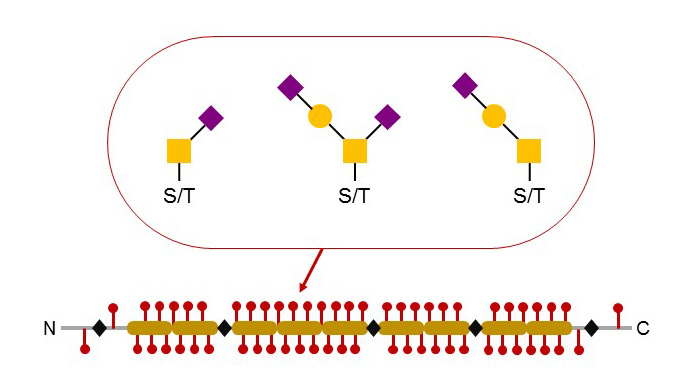
Mucins are a family of heavily O-GalNAc glycosylated proteins that have gel-forming properties and are involved in mucus formation. In mammals, at least 20 different mucin proteins are produced, most of them being secreted by epithelial cells in mucous glands, such as found in the intestine, airways and in the buccal cavity. Most mucins are large proteins of molecular weight exceeding 100 kDa, which often build disulfide bridged aggregates. The extensive glycosylation ensures a high hydration capacity and promotes resistance to proteolysis and acidic conditions. Thus, mucins mainly act as protective barriers of epithelial layers exposed to the outside. The typical O-GalNAc glycans found on mucins are short (di- to tetrasaccharides) and sialylated, of which the negative charge further increases the hydrophilicity of the glycoproteins.
Figure 46. Typical structure and glycosylation of secreted mucins. Several contiguous tandem repeats carry multiple O-glycosylated Ser and Thr residues. Most O-glycan chains are short and terminated by Sia. Mucins can build large aggregated through the formation of inter-molecular disulfide bridges.
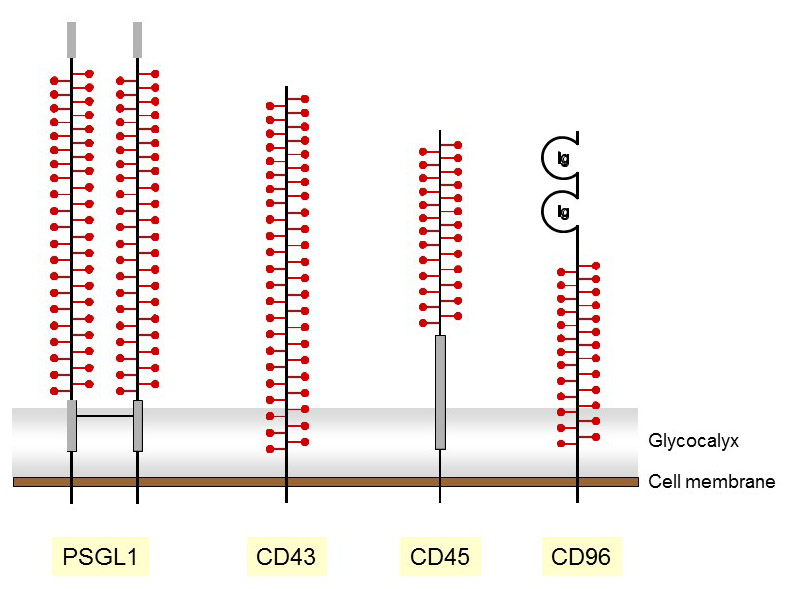
Besides the family of true mucins, several membrane proteins have mucin domains that are extensively O-GalNAc glycosylated. Several of these membrane glycoproteins are expressed on leukocytes and function as adhesion and signaling proteins. The presence of glycosylated mucin domains likely contributes to the protrusion of the corresponding glycoproteins well above the layer of glycocalyx, thereby enabling efficient interactions with ligands on other cells. These glycoproteins are often used as marker for leukocyte population, hence their naming according to the cluster of differentiation (CD) protocol. The O-glycosylation of mucin proteins is not static but changes according to the activation and differentiation status of the expressing cells. For example, the CD43 glycoprotein occurs either as a “light” 115 kDa isoform or as a “heavy” 130 kDa isoform that is found on activated T-lymphocytes. A change in O-glycosylation accounts for the difference in molecular weight. In fact, the expression of a “core 2” β1-6 GlcNAc-transferase in activated T-lymphocytes leads to the formation of heavier branched O-glycans, which leads to a shift in the molecular weight of CD43.
Figure 47. Representation of membrane mucins with O-glycosylated domains marked with red symbols.
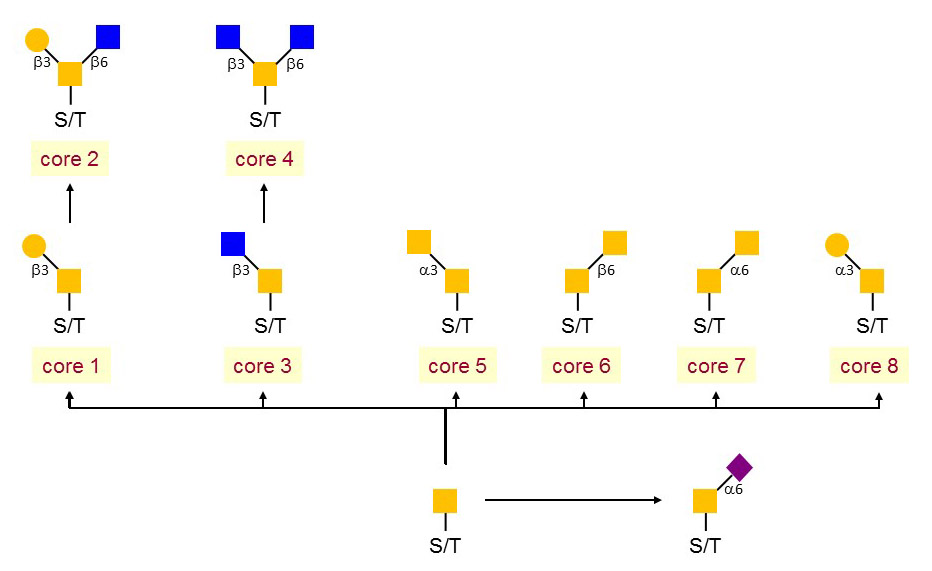
O-GalNAc glycosylation is structurally heterogeneous. Following the transfer of the core GalNAc residue to serine or threonine acceptors, several branching events mediate the formation of multiple core structures. The core 1 and core 2 structures, which are generated by the action of the core 1 β1-3 Gal-transferase and the subsequent action of the core 2 β1-6 GlcNAc-transferase, are the most frequent O-GalNAc backbones. Additional cores are formed by branching glycosyltransferases, which are mainly expressed in the gastrointestinal tract and in other tissues involved in mucin secretion. The importance of these cores in intestinal physiology has been established in mouse models, in which the branching glycosyltransferase genes have been inactivated, such as the core 3 β1-3 GlcNAc-transferase gene. The formation of O-GalNAc core structures can be prevented by the transfer of α2,6 Sia on the first GalNAc residue, thereby yielding to the so-called sialyl-Tn antigen.
Figure 48. Mucin-type core structures.
A multitude of glycosyltransferases localized in the Golgi apparatus elongate O-GalNAc glycans. The transfer of the first GalNAc is catalyzed by a large family of polypeptide GalNAc-transferases, which comprise 15 to 20 distinct isoforms. The unique core 1 β1-3 Gal-transferase is the exception to the rule of multiple glycosyltransferase isoforms acting along the elongation pathway of O-GalNAc glycans. The glycosyltransferases involved in the transfer of intermediate and terminal sugars are not specific to O-glycans. For example, the 6 β1-4 Gal-transferases and 3 α2-3 Sia-transferases elongating the core 1 branch of O-glycans are the same enzymes as those involved in the glycosylation of N-glycans. The core 2 branch is not terminated by β1-6 linked GlcNAc, but usually further elongated by poly-N-acetyllactosamine repeats or by sialylation.
The family of polypeptide GalNAc-transferases encompasses about 20 isoforms as deduced by sequence homology searches in genomic databases. Not all the putative polypeptide GalNAc-transferase genes have been confirmed to encode true enzymes and some may indeed represent pseudogenes. The polypeptide GalNAc-transferase genes are expressed in a tissue and developmental stage manner. Some isoforms, like the polypeptide GalNAc-transferase-1, are expressed nearly pleiotropically, others are only expressed in specific cell types. The recognition of peptide acceptors by the polypeptide GalNAc-transferase isoforms adds another level of complexity to the regulation of O-GalNAc glycosylation. Whereas some isoforms show little acceptor specificity, others recognize specifically glycopeptides that already carry O-GalNAc residues in proximity to the accepting serine or threonine sites. Some isoforms show strict substrate preferences, so that they only glycosylate few target proteins. Structurally, polypeptide GalNAc-transferases are type-II transmembrane proteins with a large catalytic domain and a C-terminal lectin domain, which is unique to this family of Golgi glycosyltransferases. The lectin domain, which is homologous to the B chain of ricin, participates in the recognition of acceptor (glyco)peptides. The multiple polypeptide GalNAc-transferases with their different acceptor specificities explain why a prediction of O-glycosylation sites in glycoproteins is hardly possible.
Most GalNAc cores are elongated by the addition of β1-3 linked Gal. This step is catalyzed by the core 1 β1-3 Gal-transferase enzyme in the Golgi apparatus. This enzyme is unique as it requires a specific chaperone for proper folding in the ER. This chaperone, called Cosmc, is also a type-II transmembrane protein and shows significant structural similarity to the core 1 β1-3Gal-transferase protein. A second Cosmc-like gene is expressed in few tissues like the brain, but its biological significant is still unclear. Mutations in the human Cosmc gene have been reported as the cause of a mild hematological disorder called Tn-syndrome. This acquired condition is characterized by a mosaic presentation of bare GalNAc, also called Tn-antigen, on blood cells. Such Tn-positive cells arise from hemopoietic stem cells harboring a mutated Cosmc gene. Persons with a Tn-syndrome are healthy and only present with a mild hemolytic anemia and decreased platelet and leukocyte counts.
Because of the large family of polypeptide GalNAc-transferases and their functional redundancy in the initiation of O-GalNAc glycosylation, a disease linked to polypeptide GalNAc-transferase deficiency was long considered improbable. Therefore, the identification of mutations in the polypeptide GalNAc-transferase-3 gene in cases of tumoral calcinosis came as a great surprise. Tumoral calcinosis is a rare inherited disease characterized by the deposition of bone material in soft tissues. It is related to a disorder of the calcium-phosphate metabolism. To understand the contribution of the polypeptide GalNAc-transferase-3 enzyme in the etiology of tumoral calcinosis, we first have to introduce the hormone fibroblast growth factor-23 (FGF23). Deficiency of FGF23 is the primary cause of tumoral calcinosis. FGF23 is a glycoprotein carrying O-GalNAc glycans at a position encompassing a subtilisin-like proprotein convertase recognition site. The O-glycan at position Thr178 prevents the proteolytic cleavage of FGF23 and thereby its inactivation. The polypeptide GalNAc-transferase-3 enzyme is apparently essential for the O-glycosylation of FGF23, which explains why deficiency of either FGF23 or polypeptide GalNAc-transferase-3 yields the same disease. FGF23 is involved in the regulation of calcium and phosphate blood levels through the inhibition of the 25-hydroxyvitamin D3 1α hydroxylase activity in the kidneys. The 1α hydroxylase enzyme controls the formation of 1,25-dihydroxyvitamin D3, i.e. the active form of vitamin D and thereby the levels of calcium and phosphate in blood and tissues.