Disruption of Golgi N-glycosylation genes in mice
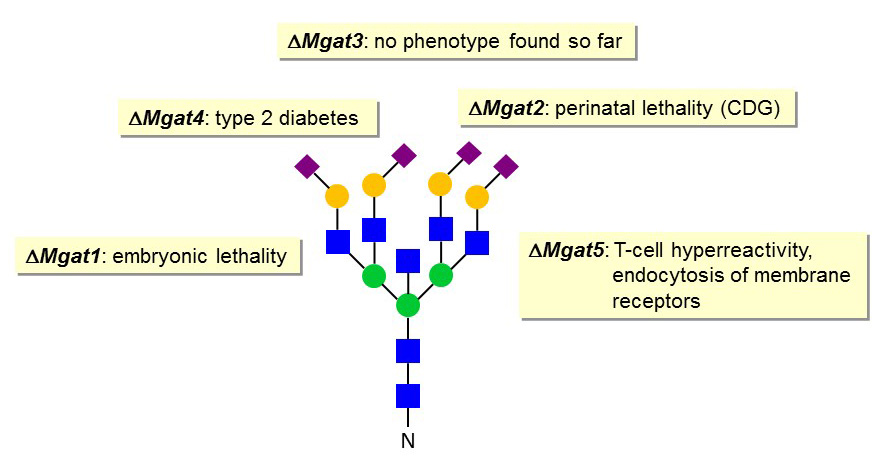
Whereas MGAT2 deficiency is the only known N-glycan branching defects in humans, nearly all branching Mgat genes have been disrupted in mice. The inactivation of Mgat1 gene leads to embryonic lethality, which is probably related to defective neural tube closure and vascularization defects. The disruption of the Mgat2 gene is associated to perinatal lethality in a mouse strain (129/Sv background) and to survival to the adult stage but with multiple organ dysfunctions in another strain (C57Black/6 background). Mgat2 knockout mice show similar phenotypes to those of MGAT2-CDG patients.
Figure 43. Phenotypes of branching defects in gene knockout mice.
The loss of the Mgat3 gene does not impair mouse development and viability. In fact, no phenotype could be identified so far in this mouse knockout model. By contrast, the inactivation of the Mgat4a gene prevents the formation of tri-antennary N-glycans, thereby affecting the stability of glycoproteins like the Glc transporter GLUT2 at the surface of pancreatic b-cells. The decreased presence of GLUT2 at the cell membrane results in Glc insensitivity and to a form of type 2 diabetes. A similar decreased stability of membrane glycoproteins is observed in Mgat5-knockout mice. This instability is caused by increased endocytosis due to reduced interaction of glycoproteins with the galectin lattice at the cell membrane. The alteration of membrane glycoprotein expression results in various alterations in signaling cascades such as exemplified by a T-cell hyperactivity phenotype.
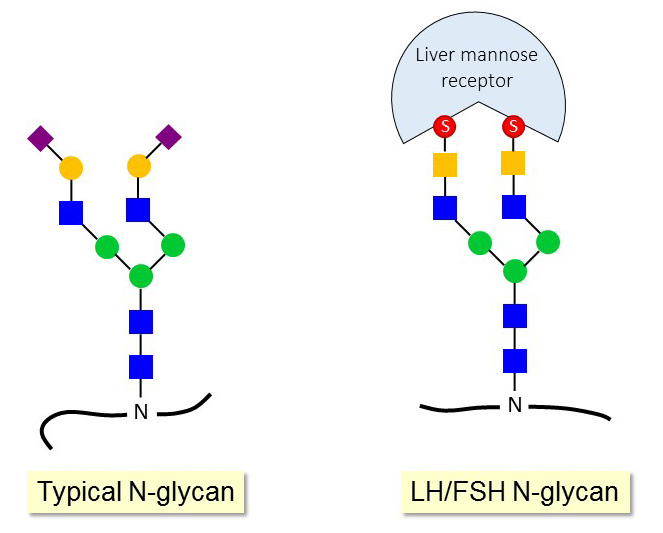
The N-glycans of the gonadotropins follicle-stimulating hormone (FSH) and luteinizing hormone (LH) carry a unique sulfated GalNAc epitope instead of Sia. The sulfated groups are essential for the binding of these hormones to the mannose receptor in the liver. The liver mannose receptor is responsible for the rapid clearance of FSH and LH from the blood circulation, which is critical to maintain a pulsatile stimulation of the gonadotropin receptors on gonad cells.
Figure 44. Sulfated GalNAc termini on gonadotropin N-glycans.
In fact, the pulsatile release of gonadotropins by the pituitary gland mediates the hormonal signal to the target cells. The pulsatile release of gonadotropins is stimulated by the peptide hormone GnRH, which is itself secreted by hypothalamic neurons in a pulsatile manner. Alterations in the pulsatile pattern of GnRH and FSH/LH lead to various hormonal disorders ranging from the shut-down of gonadotropin production to abnormal gonadal and gamete development.
The inactivation of the GalNAc-4-sulfotransferase gene in mice impairs the clearance of FSH and LH through the liver mannose receptor, which leads to elevated levels of the gonadotropins in blood. The increased gonadotropin concentrations affect the production of the sexual hormones testosterone, estadiol and progesterone and thereby the development and function of gonads and accessory glands. For example, female mice lacking the GalNAc-4-sulfotransferase gene are constantly ovulating, which also yield litters larger than average.
Another typical feature of Golgi N-glycans is the fucosylation of the GlcNAc core. This step is catalyzed by the α1-6 fucosyltransferase FUT8 gene product. Disruption of the Fut8 gene in mice (see PNAS 102, 15791-15796, 2005) and the corresponding loss of core fucosylation affects the binding of TGFβ to its receptors. This alteration of TGFβ signaling induces the expression of metalloproteinases and decreases the production of extracellular matrix proteins in lungs, which leads to pulmonary emphysema and increased lethality. The mechanisms underlying the regulation of TGFβ binding by core fucosylation are however unclear at this stage.