Oligosaccharyltransferase
The complete Dol-PP linked GlcNAc2Man9Glc3 oligosaccharide is transferred to selected Asn residues on nascent glycoproteins by the oligosaccharyltransferase (OST) complex. In the vast majority of eukaryotes, OST consists of six to eight transmembraneous proteins, which are clustered close to translocon complexes, through which nascent polypeptides enter the ER lumen. In contrast to alg glycosyltransferases that are functionally transferrable between yeast and animal cells, OST subunits are not functional when expressed across taxonomic classes.
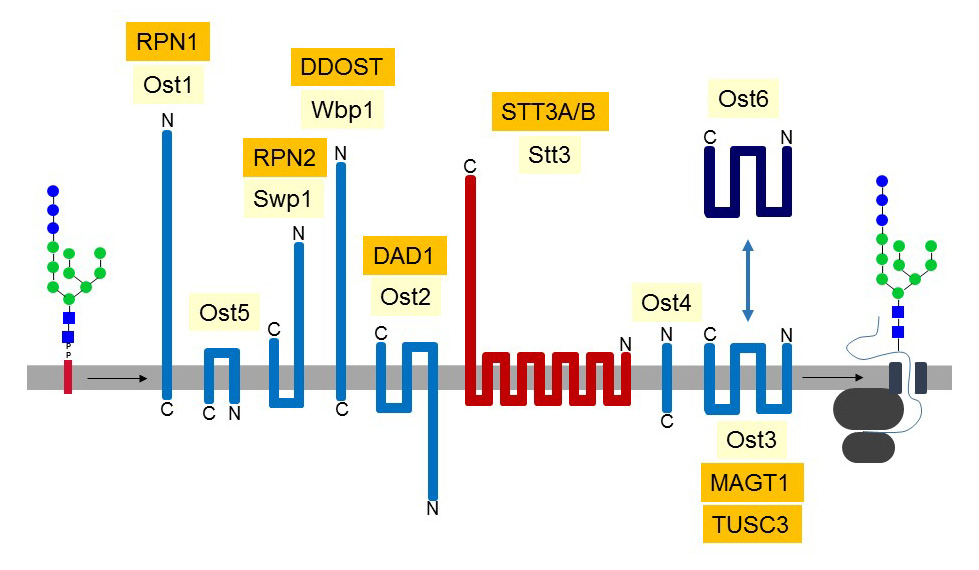
Figure 31. Symbolic representation of OST subunits characterized in the yeast Saccharomyces cerevisiae. The Ost3 and Ost6 subunits do not occur together in the same OST complex. The abbreviations of the known mammalian orthologs are marked in orange. Yeasts express a single Stt3 subunit, whereas two variants (STT3A and STT3B) are found in animals. The PglB protein of Campylobacter lari, which is orthologous to eukaryotic STT3, has been crystallized and shown to include 13 transmembrane domains.The STT3 subunit is the catalytic center of OST, whereas the contribution of most of the other subunits remain unclear. OST recognizes on the one hand the terminal Glc residue and the proximal Dol-PP-GlcNAc of the oligosaccharide substrate, and on the other hand the side chain of an Asn residue in the context of the sequon N-X-S/T, where X can be any amino acid but Pro. After transfer to a nascent polypeptide, the GlcNAc2Man9Glc3 oligosaccharide is readily trimmed by the glucosidase-I and –II enzymes, which cleave the 3 terminal Glc residues.
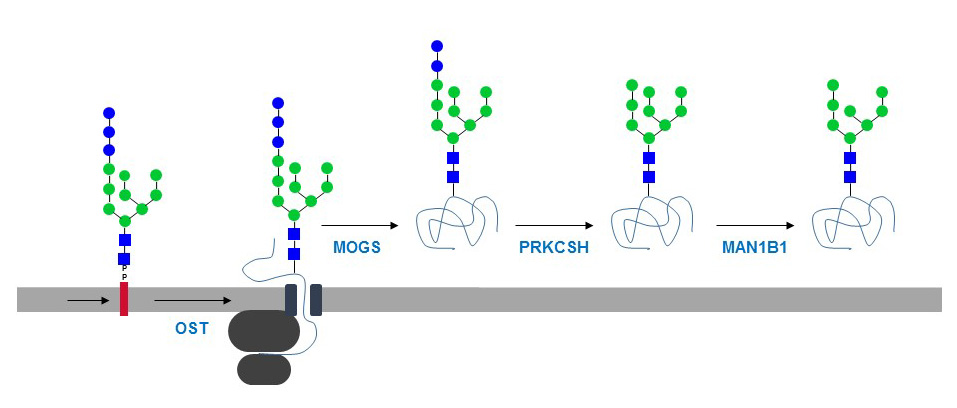
Figure 32. Transfer of complete oligosaccharide from the dolichol-PP carrier onto a nascent glycoprotein and subsequent trimming by glucosidase-I (MOGS), glucosidase-II (PRKCSH), and ER mannosidase-I (MAN1B1).
Bacterial N-linked glycosylation was first described in Campylobacter jejuni. N-linked glycosylation in Campylobacter is analog to the eukaryotic pathway in the sense that an oligosaccharide substrate is first assembled on a lipid carrier, which is then transferred by OST to Asn on acceptor polypeptides. The lipid carrier used in Campylobacter is bactoprenol-PP, which has 11 isoprenoid units (Dol has 17-24 isoprenoid units) and lacks the saturated residue carrying the primary alcohol group found in Dol. The oligosaccharide substrate in Campylobacter consists of the 7 monosaccharides GalNAc2[Glc]GalNAc3diNAcBac, where diNAcBac stands for 2,4-diacetamindo-2,4,6-trideoxy-glucose. DiNAcBac is derived from GlcNAc and shares with GlcNAc the 2-acetamido group, which is essential for the recognition of the oligosaccharide substrate by OST. Campylobacter OST is encoded by the pglB gene a functions as a single protein localized in the plasma membrane, which mediates the glycosylation of acceptor proteins in the periplasm. The PglB protein is orthologous to the STT3 subunit of eukaryotic OST. The acceptor sequon is similar to that of eukaryotes but requires Glu or Asp at -2 position, thus making: D/E-X-N-X-S/T. As in eukaryotes, X can be any amino acid but Pro.
In archaea, different oligosaccharide donors are assembled on Dol-P or Dol-PP and transferred by the OST AglB to variable acceptor sequons including N-X-S/T, N-X-N/L/V, and N-X-X-S. Recently, another type of N-linked glycosylation has been described in Haemophilus influenza and Actinobacillus pleuropneumoniae. The acceptor sequon N-X-S/T is conserved, but the monosaccharides Glc and Gal are transferred to acceptor proteins in the cytoplasma by a soluble OST enzyme.