Intracellular functions of N-glycans
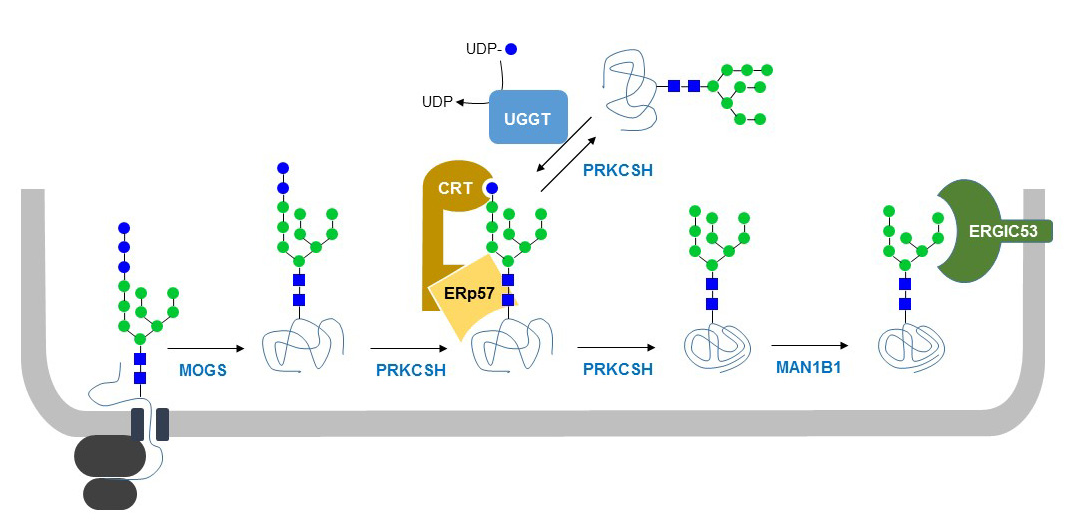
Immediately after transfer to Asn residues on glycoproteins, membrane anchored glucosidase-I trims oligosaccharides to GlcNAc2Man9Glc2. During glycoprotein folding, the second and third Glc residues are cleaved by the soluble glucosidase-II enzyme whereas soluble UDP-Glc:glycoprotein Glc-transferase (UGGT) transfers back a Glc residue to GlcNAc2Man9 on misfolded glycoproteins. This cycle of glucosylation-deglucosylation is part of the quality control of glycoprotein folding and enables interaction of misfolded glycoproteins with the Glc-specific lectins calnexin and calreticulin. These lectins are associated with the thiol-disulfide oxidoreductase ERp57, which assist in refolding glycoproteins.
Figure 33. Quality control of N-glycoprotein folding in the ER. Mono-glucosylated N-glycoproteins interact with the lectins calreticulin (CRT) and calnexin (CNX, not shown), which also bind to the oxidoreductase chaperone ERp57. Glucosidase-II (PPKCSH) cleaves the terminal Glc residue on folded and unfolded proteins. The latter are re-glucosylated by UDP-Glc:glycoprotein Glc-transferase (UGGT), which enables CRT/CNX binding and additional rounds of ERp57-mediated folding. After cleavage of the inner terminal Man residue, folded N-glycoproteins are bound by trafficking proteins such as ERGIC53 and transported out of the ER.
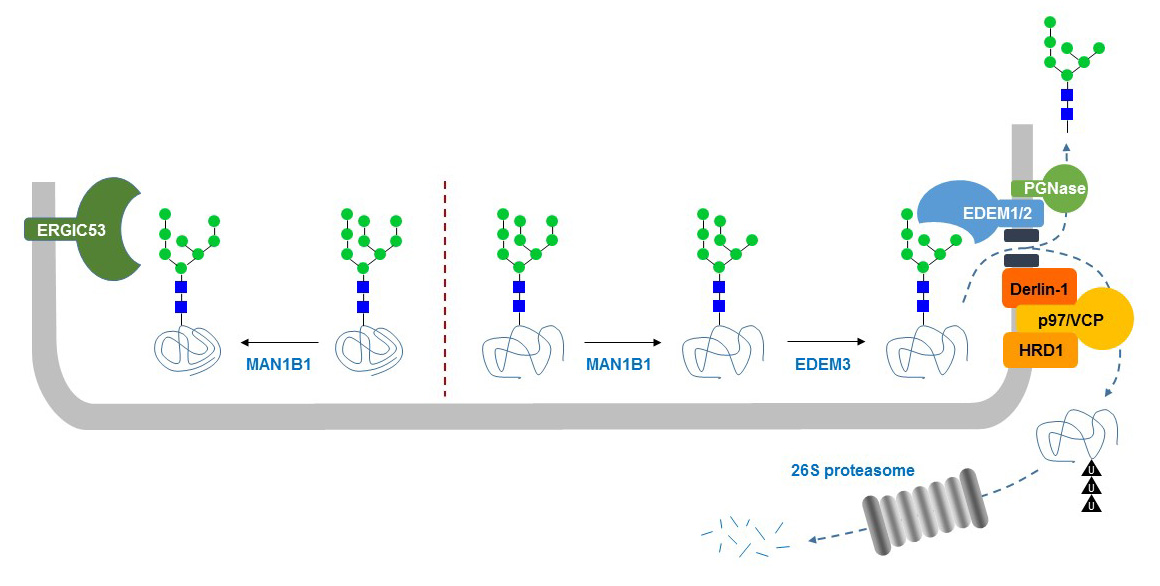
The transition from GlcNAc2Man9 to GlcNAc2Man7 represents another signal for the intracellular trafficking of glycoproteins. The ER mannosidase-I enzyme cleaves the terminal Man residue of the inner branch of N-glycans, yielding GlcNAc2Man8. Properly folded glycoproteins will leave the ER carrying this GlcNAc2Man8 glycan. If the glycoprotein remains unfolded, a second mannosidase called HTM1 in yeasts (EDEM1 to EDEM3 in mammals) will cleave terminal Man from the outer α1-6 linked branch yielding GlcNAc2Man8, which is recognized by the Yos9p lectin (OS-9 in mammals). Yos9p/OS-9 is associated with the HRD ubiquitin ligase complex involved in the retro-translocation of misfolded proteins out of the ER and of their degradation by the proteasome complex in the cytosol.
Figure 34. ER-associated protein degradation (ERAD) of misfolded N-glycoproteins.After leaving the ER, glycoproteins are transferred to the ER-Golgi intermediary compartment (ERGIC). This process is in part mediated by the N-glycan lectin ERGIC53, which is also abbreviated LMAN1 for lectin, mannose-binding, 1. Deficiency of ERGIC53/LMAN1 causes a bleeding disorder (OMIM 227300) characterized by the defective secretion of coagulation factors V and VIII, which are N-linked glycoproteins. N-glycans also carry the Man-6-P signal mediating the targeting of proteins to lysosomes. This lysosomal targeting signal is produced in two steps, consisting first of the addition of phospho-GlcNAc to Man residues on N-glycans by the Golgi-localized GlcNAc-1-phosphotransferase complex. This enzyme is a hexamer comprising two α-, two β-, and 2 γ-subunits, where the α- and β-subunits are catalytically active and ensure the recognition of the lysosomal protein substrates. The contribution of the g-subunits are unclear, but required for the full activity of the complex. Mutations in the GNPTAB gene encoding the polypeptide yielding the α- and β-subunits cause I-cell disease (OMIM 252500) and pseudo-Hurler polydystrophy (OMIM 252600), which are also classified as mucolipidosis II and mucolipidosis III. Mutations in GNPTG encoding the γ-subunit cause a variant form of mucolipidosis III (OMIM 252605). After transfer of phospho-GlcNAc to Man, an uncovering enzyme localized in the trans Golgi network removes GlcNAc, thereby leaving the phosphate group at the 6’-position of Man. The Man-6-P signal is recognized by cognate Man-6-P receptors, which capture the glycoproteins bearing the signal and transports them to the lysosomes. The presence of Man-6-P receptors at the plasma membrane enables the treatment of defective lysosomal enzymes by intravenous administration of Man-6-P bearing recombinant enzymes.