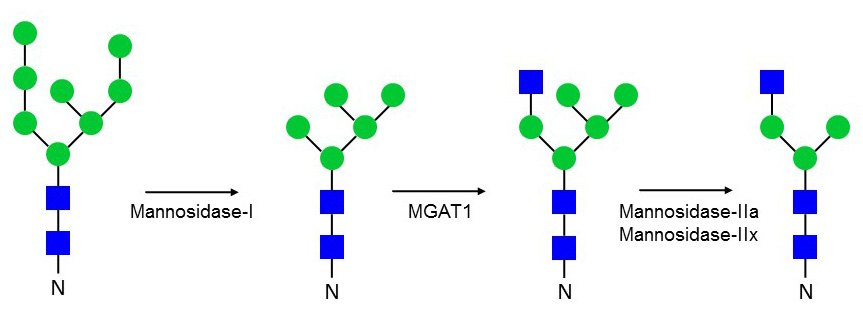
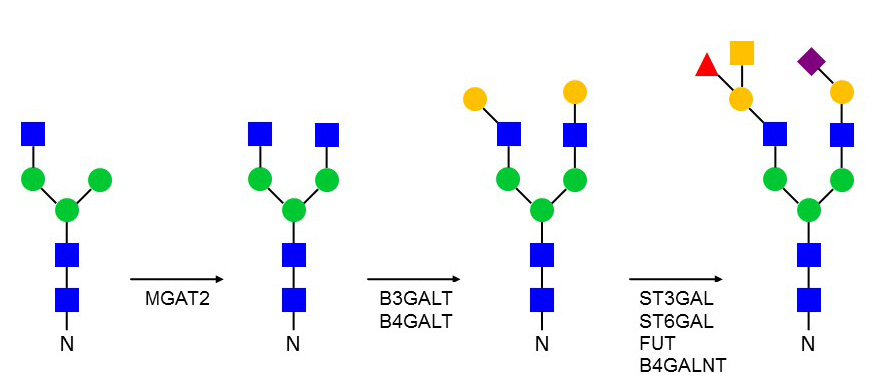
Golgi N-linked glycosylation
Whereas the pathway of N-linked glycosylation proceeds sequentially in the ER, the modifications of N-glycans in the Golgi follow parallel and alternative pathways, which contribute to the formation of variably branched and extended structures. The GlcNAc2Man8 N-glycan entering Golgi can remain unchanged during transit and exit as high-mannose core structure. Alternatively, this core can be reduced down to three Man residues by mannosidases and elongated in various ways depending on the availability of glycosyltransferases and of donor substrates. Before trimming, N-glycans can be modified by addition of a phosphate group at the 6’ position of selected mannose residues. Glycoproteins carrying N-glycans with such Man-6-P residues are recognized by the Man-6-P receptor in the Golgi and transported to lysosomes.
The first trimming step in the Golgi apparatus is performed by mannosidase-I enzymes, which cleave the two α1-2 linked Man sitting on the α1-3 linked Man branch. This step is necessary to enable further modifications, such as the addition of a β1-2 linked GlcNAc to α1-3 linked Man. The branching GlcNAc-transferases are abbreviated MGAT for mannoside glycoprotein N-acetylglucosaminyl transferase. Accordingly, MGAT1 transfers the first branching β1-2 GlcNAc to Man. This GlcNAc residue can be further elongated by Gal-, Fuc- and Sia-transferases, thereby giving rise to so-called hybrid N-glycans. The next Man trimming follows the addition of β1-2 GlcNAc by MGAT1. Mannosidases-IIa and –IIx perform this trimming of the α1-3 and α1-6 linked Man residues. Both mannosidases-II share the same substrate specificity but are expressed in different tissues. The combined deficiency of mannosidases-IIa and –IIx is embryonic lethal in mice and precludes the formation of complex N-glycans.
Figure 37. Trimming of high-mannose N-glycans in the Golgi apparatus.
After mannosidase trimming, a second β1-2 GlcNAc is added to the α1-6 linked Man by the MGAT2 enzyme. N-glycans comprising at least two elongated GlcNAc branches are called complex N-glycans. Also the first GlcNAc that is attached to Asn can be modified by addition of a α1-6 Fuc. The elongation of GlcNAc branches is variable since it depends on glycosyltransferases, which are expressed in developmental stage- and tissue specific patterns. Some of the glycosyltransferases elongating N-glycans can also act on O-glycans and on glycosphingolipids. The structural heterogeneity of N-glycans is such that a single glycoprotein can carry complex, hybrid and high-mannose N-glycans on the same polypeptide.
Figure 38. Elongation of biantennary N-glycans in the Golgi apparatus.