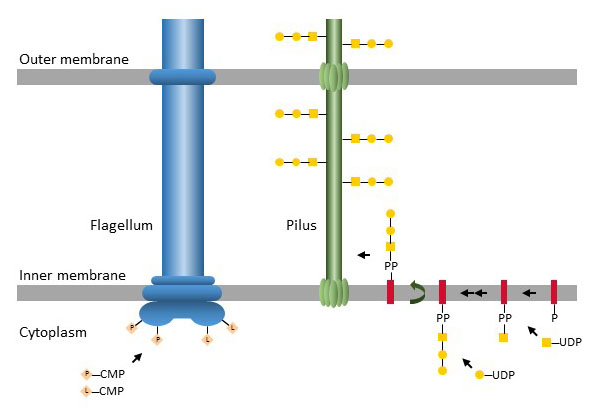
Protein O-glycosylation
Beyond polysaccharides, bacteria harbor complex glycosylation machineries modifying proteins. Some of these glycosylation pathways are specific to few target proteins, whereas others generate a broad range of glycoproteins. As in eukaryotes the amino acids Ser and Thr are the main carriers of O-glycans in bacteria, although Tyr has also been described as carrier in some Gram-positive mesophilic bacteria. Flagellum and pilus proteins are the most frequent targets of O-glycosylation pathways. The glycosylation of flagella takes place in the cytoplasm by direct glycosylation of Ser/Thr residues on the target proteins. By contrast, pilus O-glycosylation proceeds in two steps through pathways similar to O-antigen biosynthesis.
Figure X. O-glycosylation of flagellar proteins by cytoplasmic glycosyltransferases and en bloc transfer of oligosaccharides to Ser/Thr of pilus proteins. P: pseudaminic acid, L: legionaminic acid.
A large variety of monosaccharides are transferred to flagellar proteins across bacteria. For example, Campylobacter jejuni heavily glycosylates flagella with the ulosonic acids (C9 sugars) pseudaminic acid (Pse), legionaminic acid (Leg) and derivatives. Pseudomonas aeruginosa glycosylates its flagella with Rha-based oligosaccharides. O-Glycosylation is required for the assembly of flagella and hence for bacterial motility. The oligosaccharide chains transferred en bloc to pili proteins also vary in composition and size across bacterial groups. Some strains of Pseudomonas aeruginosa transfer an oligosaccharide derived from the LPS O-antigen pathway, whereas other strains have developed a specialized pathway for pili glycosylation. The O-glycosylation machinery of some bacteria, such as Neisseria and Bacteroides spp., is not restricted to flagella and pili. Several surface proteins have been identified as carrying O-glycans, although the general significance of such modifications remain unclear. Among other effects, O-glycosylation has been postulated to improve bacterial viability and decrease recognition by host immune cells. Some bacterial O-glycosyltransferases are structurally and functionally similar to eukaryotic counterparts. Man-transferases found in Streptomyces and Mycobacterium spp. use (un)decaprenol-P-Man as donor substrate to glycosylate proteins with O-Man. These bacterial Man-transferases share structural similarity to eukaryotic PMT enzymes. A paralog to eukaryotic O-GlcNAc-transferase (OGT) has also been identified in Listeria monocytogenes and shown to be involved in O-GlcNAc-ylation of flagellar proteins.