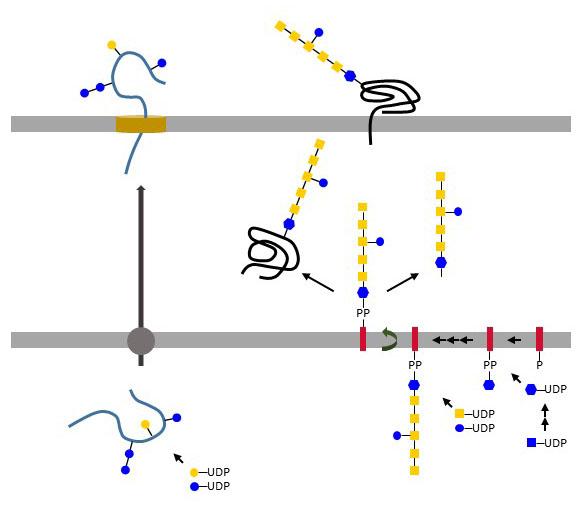
Protein N-glycosylation
N-glycosylation has been best characterized in Campylobacter jejuni, in which it was also first described. The similarity between Campylobacter and eukaryotic N-glycosylation pathways is striking. In both cases, the pathway begins at the cytoplasmic leaflet of the cell membrane with the assembly of an oligosaccharide on a polyprenol-PP carrier. This polyprenol-PP-oligosaccharide flips across the membrane and the oligosaccharide is transferred en bloc to Asn residues in the context of the sequon Asn-X-Ser/Thr. The true consensus site for N-glycosylation in Campylobacter jejuni is in fact more stringent as it requires an acidic amino acid, i.e. Glu or Asp, at position -2 of the acceptor Asn, thus yielding Glu/ Asp-X-Asn-X-Ser/Thr where X cannot be Pro. The oligosaccharide synthesized in Campylobacter on undecaprenol-PP is an heptasaccharide featuring the GlcNAc derivative 2,4-diacetamido-2,4,6-trideoxy-a-D-Glc (diNAcBac), GalNAc and Glc. After translocation to the periplasmic side of the membrane, the oligosaccharide is cleaved from undecaprenol-PP and transferred to acceptor proteins by the membrane-embedded oligosaccharyltransferase PglB. In contrast to eukaryotic oligosaccharyltransferases, PglB acts on folded proteins and shows a relaxed specificity for the oligosaccharide transferred as long as the monosaccharide at the reducing end contains an acetamido group at C2. Furthermore, the majority of the oligosaccharides cleaved by PglB are not transferred to proteins but remain as free oligosaccharides in the periplasm. The general significance of N-glycosylation in Campylobacter jejuni is unclear. Mutants lacking N-glycosylation show various phenotypes, such as impaired proliferation in animals and decreased adhesion to host tissues. It is likely that functional impairments are related to some of the 100 N-glycoproteins identified in Campylobacter jejuni. Oligosaccharyltransferase-like proteins similar to PglB are found in different bacterial families including Desulfovibrio and Helicobacter spp., although not in Helicobacter pylori. N-glycosylation also occurs in Archaea, in which various oligosacchrides are transferred to acceptor S-layer glycoproteins after assembly on dolichol-PP as in eukaryotes. The archeal oligosaccharyltransferase AglB recognizes the sequon Asn-X-Ser/Thr but does not required an N-acetylated aminosugar at the reducing end of the oligosaccharide. N-Glycosylation of S-layer proteins contribute to the resistance of Archaea to hostile conditions. Interestingly, the halophile Haloferax volcanii changes the N-glycosylation pattern of its S-layer proteins based on the salinity of the environment.
Figure X. Direct N-glycosylation of proteins in Haemophilus influenzae and Actinobacillus pleuropneumoniae (left side) and block-wise transfer of oligosaccharides by PglB oligosaccharyltransferase in Campylobacter jejuni (right side).
Another type of N-glycosylation involving the sequential transfer of monosaccharides on proteins has been recently described in Haemophilus influenzae and Actinobacillus pleuropneumoniae. there, cytoplasmic glycosyltransferases attach Glc or Gal from UDP-activated substrates to Asn in the typical Asn-X-Ser/Thr context. The few examples outlined here are probably the tip of the iceberg and future work will certainly further expand the complex chapter of bacterial glycosylation.