Bacterial polysaccharides
Bacteria produce and secrete several types of oligo- and polysaccharides, which contribute to the formation of capsules and biofilms. Capsular polysaccharides increase the survival of bacteria in hostile environments, and are accordingly classified as virulence factors for pathogenic bacteria. Most of these polysaccharides are poorly immunogenic and further prevent the activation of complement-mediated lysis and phagocytosis. Some capsular polysaccharides, such as polysialic acid and hyaluronan, even mimic host glycans and thereby evade immune recognition.
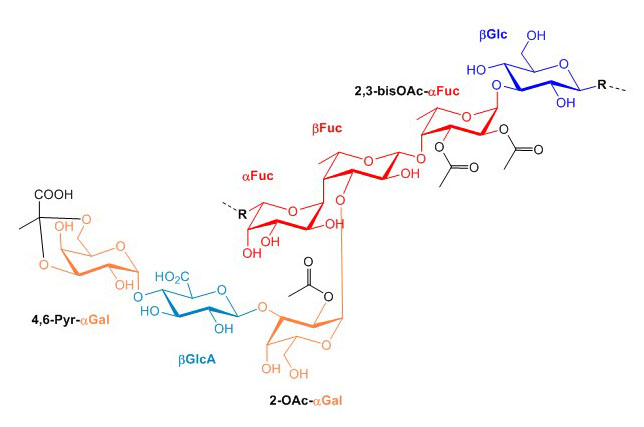
The polysaccharide colanic acid, also known as M-antigen, was originally isolated from Escherichia coli but is also found covering other Enterobacteriaceae. The core of colanic acid consist of seven monosaccharides including Gal, GlcA, Glc, and Fuc. Colanic acid heptasaccharides are built at the cytoplasmic side of the inner membrane on the carrier undecaprenol-PP, then flipped to the periplasm, where multiple colanic acid units are polymerized and secreted in the extracellular space. Colanic acid is sometimes attached to the O-antigen of LPS. The synthesis of colanic acid is turned on in response to environmental stress related to damage of cell envelope.
Figure X. Core structure of colanic acid from Escherichia coli K12. Connection points for the polymerization of colanic acid units are marked with R.
The utilization of polyprenol-PP as a carrier in the synthesis of glycoconjugates is a recurrent feature across glycosylation pathways in prokaryotes and eukaryotes. Dolichol-PP is used in eukaryotes and Archaea, and bactoprenol-PP is used in bacteria for the synthesis of multiple structures including colanic acid, peptidoglycan, teichoic acid, lipopolysaccharide, lipoarabinomannan, N-linked glycans, and O-linked glycans. The translocation of lipid-linked oligosaccharides across membranes is also common to many glycosylation pathways.
The main types of large capsular polysaccharides are polysialic acid, hyaluronan, and poly β1-6 GlcNAc. Polysialic acid consists of polymers of α2-8 and α2-9 linked Sia and occurs on pathogenic Escherichia coli, Neisseria meningitides and Pasteurella hemolytica. As for all other types of sialylation in bacteria, only Neu5Ac is found in polysialic acid chains. The biosynthesis of polysialic acid takes place at the cytoplasmic side of the inner membrane using CMP-Sia as donor substrate. The acid polymer transits then through a multiprotein channel across the periplasm to be secreted in the extracellular space. Escherichia coli and some strains of Neisseria meningitides. In addition to promoting immune evasion by mimicry, polysialic acid inhibits complement activation and phagocytosis.
Figure X. Biosynthesis of colanic acid at the cytoplasmic leaflet of the inner membrane on the bactoprenol undecaprenyl-PP. Colanic acid units polymerize after flipping to the periplasmic space.
Hyaluronan is another virulence factor contributing to the pathogenicity of bacteria such group A Streptococcus. Bacterial hyaluronan is, like its counterpart in animals, produced at the cell membrane by sequential elongation of a hyaluronan chain from the reducing end. Capsular hyaluronan shields bacteria from immune surveillance and confers resistance to phagocytosis. The last type of large polysaccharides found in bacteria is poly-N-acetylglucosamine, commonly abbreviated PNAG, which is produced by certain gram-positive and gram-negative bacteria. Such chains of poly β1-6 GlcNAc are for example deployed by pathogenic Escherichia coli for biofilm formation under limiting carbon availability.