Navigation auf uzh.ch
Navigation auf uzh.ch
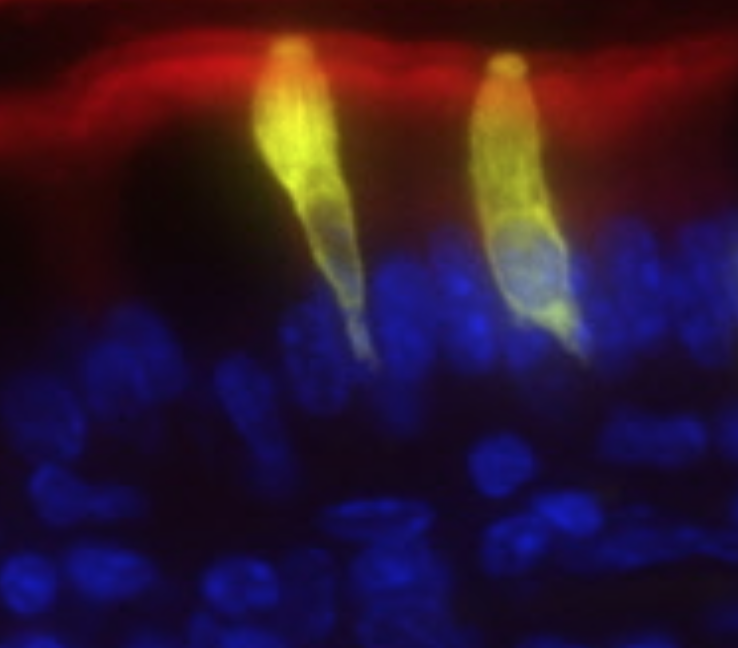
The goals of this project are the identification of novel factors regulating tuft cell functions, their roles in physiology, and the mechanisms of tissue remodeling during intestinal worm infection.
Tuft cells are rare, secretory epithelial cells that generated scant immunological interest until recent reports linked tuft cells with type 2 immunity in the small intestine. The small intestinal tuft cell–ILC2 circuit mediates epithelial responses to intestinal worms and protists by tuft cell gustatory sensing and IL-25-mediated activation of IL-13 secretion by group 2 innate lymphoid cells (ILC2s).
Tuft cells have emerged as potential central players in tissue-immune cross-talk due to their capacity to sense luminal signals, including metabolites (Schneider C. et al., 2018, Cell), and to produce an unusual spectrum of biological effector molecules, including IL-25, eicosanoids, and the neurotransmitter acetylcholine. Despite recent insights, numerous questions remain regarding tuft cell lineage, diversity and effector mechanisms and how tuft cells interface with the immunological niche in the tissues where they reside, offering exciting and novel research avenues.
The aim of this project is to identify the pathways involved in small intestinal adaptive remodeling, which is induced by intestinal worm infection. We try to dissect the roles of tuft cells and other type 2 immune components in this process by using in vivo mouse models combined with lineage-tracing of intestinal stem cells. Using a combination of in vivo and in vitro approaches, and by employing cytokine reporter mice and organoid systems, we aim to identify novel factors regulating tuft cell function and their roles in intestinal physiology.
Picture: The small intestinal tuft cell–ILC2 circuit. Schneider C, O’Leary CE & Locksley RM, Nat Rev Immunol (2019) Copyright © 2019, Springer Nature).
In this project, we aim to dissect components of the immune – tissue cross-talk in the context of alveolar macrophage niche establishment, and its impact on lung function in homeostasis and during pulmonary inflammation.
Tissue-resident immune cells play key roles in organ physiology by their cross-talk with non-immune cells in the tissue niche. The perinatal period is a critical window for distribution of innate tissue-resident immune cells within developing organs, where they acquire a tissue-specific gene expression signature that is associated with their distinct function, as recently described for ILC2s and alveolar macrophages by us and others (Schneider C. et al, Immunity, 2019; Schneider C. et al, Nat. Immunol., 2014). A focus of our laboratory is to understand how this tissue–immune cross-talk contributes to tissue homeostasis. In particular, we are interested in the organization of the alveolar macrophage niche, the individual contribution of different immune and non-immune cells to this environment, and how local alterations are induced in the context of pulmonary type 2 inflammation and influenza infection with impact on lung function.
These studies are enabled by the unique tools available in our lab, including novel reporter and conditional KO mice, which allow the tracking and manipulation of the relevant cell types in situ. We use various mouse in vivo techniques, in vitro organoid culture systems, and in tissue cell characterization using multi-parameter flow cytometry, advanced imaging, and RNA-seq.