Navigation auf uzh.ch
Navigation auf uzh.ch
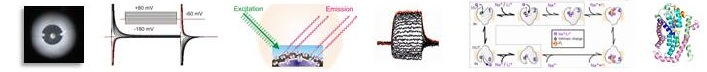
2010 - 2015
We continued investigation of the structure-function relations of sodium-coupled phosphate transporters (NaPi-II, SLC34 family) previously started by the Murer group. We focused on the voltage clamp fluorometry technique, combined with high resolution determination of charge relaxations recorded from oocytes over-expressing constructs containing point mutations. With these experimental tools, we gained important insights into substrate interactions and voltage dependence of transport function. In the absence of a crystal structure for bacterial homologs of SLC34 proteins, we also adopted a homology modelling strategy in collaboration with US colleagues, using the bacterial VcINDY dicarboxylate transporter as template. This work, complemented with experimental studies yielded a 3-D structural model for SLC34 proteins and allowed us to locate the substrate and cation binding sites and predict molecular rearrangements during the transport cycle.
|
Head |
Members |
|
|
Monica Patti |